European citizens need access to innovative and affordable healthcare products and services. Effective competition means that companies producing pharmaceuticals, healthcare devices, or other health-related products and services compete with one another based on quality and price.
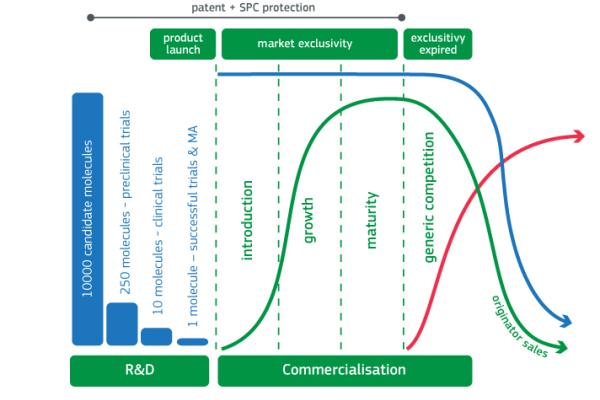
The development cycles for innovative drugs are usually risky and lengthy and entail high development costs. Certain practices carried out by pharmaceutical companies to protect their exclusivity may, under certain circumstances, lead to violations of EU competition law. These include, for example, patent clusters, patent thickets and patent settlements, which aim at prolonging market-exclusivity.
The Commission’s main enforcement actions in antitrust and mergers in the pharmaceutical sector are described in its updated Report on competition enforcement in the pharmaceutical sector 2018-2022 (available in DE/EN/FR and all other EU languages). The Report was drawn up in close co-operation with the national competition authorities of the EU Member States. It explains how antitrust and merger decisions adopted by national competition authorities and the Commission during that period contributed to affordable and innovative medicines for EU patients.