Cases
Antitrust
The Commission monitors agreements between companies and the conduct of dominant players and may take action against them if it has concerns that they are anticompetitive. If these concerns are confirmed, and the EU antitrust rules (Articles 101 and 102 TFEU) are breached, the Commission may adopt decisions ordering the companies concerned to stop and desist from the infringing conduct and imposing fines.
Enforcement decisions
The Commission has adopted a number of individual decisions against pharmaceutical companies following a breach of EU Antitrust rules:
Excessive Pricing
In 2021, the Commission accepted commitments from Aspen to reduce prices for six off-patent medicines by 73%, addressing excessive pricing concerns.
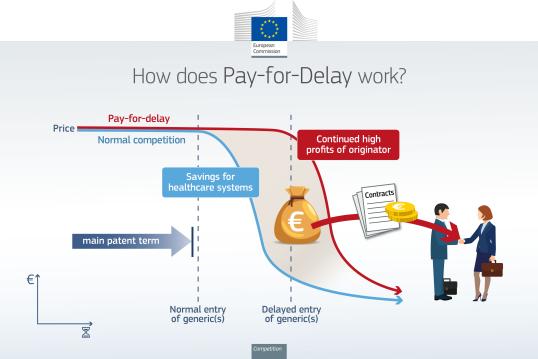
Delaying the market entry of generic medicines
In 2020, the Commission fined Teva and Cephalon a total of €60.5 million for agreeing to delay for several years the market entry of a cheaper generic version of Cephalon's drug for sleep disorders, modafinil.
In 2014, the Commission fined Servier €330 million, and five producers of generic medicines €97 million for concluding a series of deals all aimed at protecting Servier's bestselling blood pressure medicine, Perindopril, from price competition by generics in the EU. Servier appealed the Commission’s Decision, which was partially annulled by the General Court in 2018. Servier further appealed the Decision in 2019, which is still pending.
In 2013, the Commission fined Johnson & Johnson and Novartis €16 million for delaying market entry of generic pain-killer, Fentanyl. Also in 2013, Lundbeck was fined €93.8 million along with several producers of generic medicines who were fined a total of €52.2 million for delaying the market entry of generic antidepressant citalopram
In 2005, the Commission fined AstraZeneca €60 million. This followed an investigation by the Commission into AstraZeneca's suspected abuse of the patent system and the system for authorisation of medicines, with the aim of delaying competition to a blockbuster drug from generic and parallel imported pharmaceuticals. The abuse of Astra Zeneca consisted of providing misleading information to a number of EEA patent offices in order to obtain supplementary protection certificates (SPCs) and misusing rules and procedures applied by national medicines agencies by selectively deregistering the market authorisations. In 2010, the General Court largely upheld the Commission’s decision, but lowered the fine from €60 to €52.5 million. AstraZeneca’s further appeal was rejected by the European Court of Justice in 2012.
Health Services
The Commission adopted its first antitrust decision in the Health Services sector in 2010. It imposed a fine of EUR 5 million on the Ordre National des Pharmaciens for imposing minimum prices on the French market for clinical laboratory tests, and hindering the development of laboratory groups.
Mergers
The Commission prevents mergers that are likely to harm competition in the healthcare sector. Most mergers are cleared under the simplified procedure, or following an in-depth investigation. Some mergers are cleared only after the merging parties have committed to corrective actions.
Since the 1980’s, the concentration process in the pharmaceutical industry has mainly concerned multinationals. It is important to ensure that a new concentration neither impedes generic competition, in the case of Research and Development and generic companies or two generic companies merging, nor suppresses competition in R&D, when two R&D companies are concerned.
In May 2021, the Commission issued a fining decision against Sigma-Aldrich for providing misleading information during the Commission’s investigation under the EU Merger Regulation of Merck’s acquisition of Sigma-Aldrich. This was the third time that the Commission had issued a fining decision under the EU Merger Regulation of 2004, and the first time within the sector for pharmaceutical products.
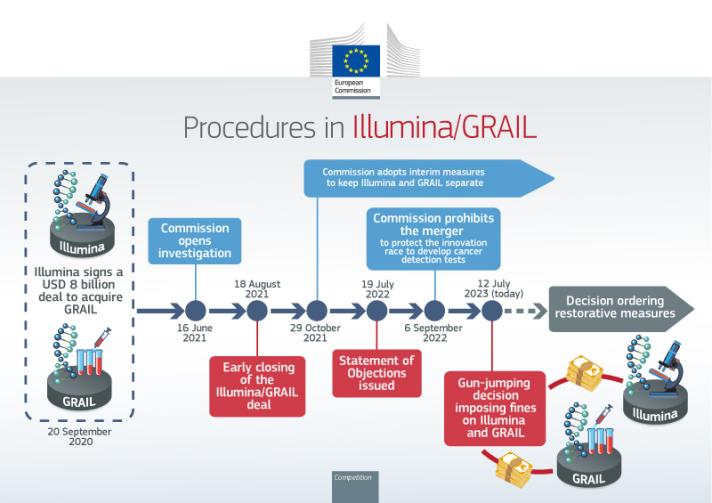
The Commission opened its investigation into Illumina’s acquisition of GRAIL on 16 June 2021 after having accepted requests from several Member States to refer the merger to the Commission under Article 22 of the EU Merger Regulation. On 13 July 2022, the General Court confirmed the Commission’s jurisdiction to review the transaction pursuant this referral. On 6 September 2022, following its in-depth investigation, the Commission prohibited the acquisition of GRAIL by Illumina. The Commission concluded that the transaction would have stifled innovation and reduced choice in the emerging market for blood-based early cancer detection tests. Illumina did not offer remedies sufficient to address these concerns.
This prohibition decision came after Illumina and GRAIL completed the transaction in August 2021, while the Commission’s investigation was still ongoing. Therefore, in order to give effect to the prohibition decision, on 5 December 2022, the Commission sent a Statement of Objections to Illumina and GRAIL informing them of the restorative measures it intends to adopt under the EU Merger Regulation in order to unwind Illumina’s blocked acquisition of GRAIL. The Statement of Objections sets out the proposed divestment and transitional measures that Illumina and GRAIL need to comply with until the transaction has been dissolved. The transitional measures will replace the interim measures that were renewed and adjusted by the Commission on 28 October 2022.
In addition, on 19 July 2022, the Commission sent a Statement of Objections to Illumina and GRAIL alleging on a preliminary basis that the implementation of the transaction while the Commission’s investigation was ongoing was in breach of the ‘standstill obligation’ under Article 7 of the EU Merger Regulation, which may ultimately lead to the imposition of a fine. The Commission will consider Illumina’s and GRAIL’s responses to these two Statement of Objections before reaching its final decision on the respective points.
On 12 July 2023, the Commission confirmed its preliminary view that Illumina and GRAIL intentionally breached the standstill obligation and issued fines against both Illumina and GRAIL. The Commission found that by closing the transaction, Illumina was able to exercise a decisive influence over GRAIL and it actually exercised it. According to the EU Merger Regulation the Commission can impose fines of up to 10% of the aggregated turnover of companies, which intentionally or negligently breach the standstill obligation. In the case of Illumina this translates to a fine of approximately €432 million. In the case of GRAIL, the Commission decided to impose only a symbolic fine of €1,000 as this is the first time it imposes a fine for gun-jumping on a target company.
Health Services
The Commission has examined a limited number of mergers in the health care services sector. Most have been cleared using the simplified procedure since they did not raise competition concerns.
In April 2012 the Commission cleared, with conditions, the proposed acquisition of Synthes Inc. by Johnson & Johnson, both US companies active in the area of orthopaedic medical devices. The Commission's investigation confirmed that, subject to the divestment of Johnson & Johnson's trauma business, the merged entity would continue to face competition from a number of other strong competitors and that customers would still have sufficient alternative suppliers in all of the markets concerned.
These cases highlighted the main challenges for national authorities: how to establish transparent entrustment acts which precisely define public services and their public funding, and accurately separating accounts between public and commercial services. The Commission required appropriate amendments where necessary.
State Aid
The Commission monitors and may intervene in relation to State measures that grant unfair advantages to companies active in the healthcare sector, thereby ensuring pharmaceuticals markets offer a level-playing field to companies. State aid investigations have also included a number of complaints lodged by private health service providers against their allegedly unfair treatment or against potential excessive compensation of publicly-owned hospitals.
The Commission has adopted a number of decisions allowing Member States to give aid in the field of pharmaceuticals. The aid has taken the form of tax exemptions or direct grants to enterprises in the framework of regional aid, allowing for investments in eligible regions which are lagging behind economically.
The Commission also examines cases involving possible state aid in the field of health insurance, in particular in countries with competitive health insurance markets.
The State Aid Temporary Framework
The State aid Temporary Framework and public support of research, testing and production of products relevant to fighting the Coronavirus outbreak was adopted on 19th March 2020 to enable Member States to use the full flexibility foreseen under State aid rules to support the economy in the context of the coronavirus outbreak. On 3rd April 2020, the first amendment to the Temporary Framework increased the possibilities for public support for research, testing and production of products relevant to fight the coronavirus outbreak.
- First Amendment to the Temporary Framework to support the economy in the context of the coronavirus outbreak
(OJ C 112I, 4.4.2020, p. 1–9) - Press release (03.04.2020)
- Commission Statement on consulting Member States on the proposal to extend State aid Temporary Framework
For a full list of Decisions adopted under the State Aid Temporary Framework, including those in the pharmaceuticals sector, see Coronavirus Decisions List.
To search further Commission Decision relevant to the pharmaceutical and healthcare sectors, please use the Case Search Tool.
Judgments
Delay of entry
General Court
- Cases T-677/14, T-679/14, T-680/14, T-682/14, T-684/14, T-691/14, T-701/14, T-705/14 Servier SAS and others v Commission
Judgments of the Court of 12 December 2018 - Cases T-460/13, T-467/13, T-469/13, T-470/13, T-471/13, T-472/13 Lundbeck
Judgments of the Court of 8 September 2016
Court of Justice
- Case C-591/16 Lundbeck
Judgment of the Court of 25 March 2021 - Case C-614/16 Merck v Commission
Judgment of 25 March 2021 (rectified by order of 3 September 2021) - Case C-601/16 Arrow Group and Arrow Generics v Commission
Judgment of the Court of 25 March 2021 - Case C-588/16 Generics (UK) Ltd, v. the Commission
Judgment of 25 March 2021 - Case C-586/16 Sun Pharmaceutical Industries and Ranbaxy (UK) v Commission
Judgment of 25 March 2021 (rectified by order of 3 September 2021) - Case C-307/18 Generics (UK and Others
Judgment of 20March 2020 - Case C-457/10 P AstraZeneca v Commission
Judgment of the Court of 6 December 2012 - Cases C-207/03 and C-252/03 Novartis
Judgement of the Court of 21 April 2005 - Case C-36/03 Approved Prescription Services Ltd v Licensing Authority
Judgement of the Court of 9 December 2004 - Case C-106/01 Novartis Pharmaceuticals
Judgement of the Court of 29 April 2004 - Case C-316/95 Generics
Judgement of the Court of 9 July 1997 - Case C-238/82 Duphar BV and others v The Netherlands State
Judgement of the Court of 7 February 1984
Parallel trade
General Court
- Case T-574/14 European Association of Euro-Pharmaceutical Companies (EAEPC) v Commission
Judgment of the Court of 26 September 2018 - Case T-247/04 Aseprofar and Edifa v Commission
Order of the Court of 19 September 2005
Court of Justice
- Joined Cases C-501/06 P, C-513/06 P, C-515/06 P, C-519/06 P GlaxoSmithKline Services and others v Commission and others
Judgements of the Court of 6 October 2009 - Joined Cases C-468/06 to C-478/06 Sot. Lelos kai Sia EE and others v GlaxoSmithKline AEVE Farmakeftikon Proionton
Judgment of the Court of 1 April 2008 - Case C-348/04 Boehringer Ingelheim and Others
Judgement of the Court of 26 April 2007 - Case C-438/02 Hanner
Judgement of the Court of 31 May 2005 - Case C-112/02 Kohlpharma v Bundesrepublik Deutschland
Judgement of the Court of 1 April 2004 - Case C-201/ 94 Smith & Nephew Pharmaceuticals and Primecrown v Medicines Control Agency
Judgement of the Court of 1 April 2004 - Cases C-2/01 P and C-3/01 P BAI v Bayer and Commission
Judgment of the Court of 6 January
Other
- Case C-179/16 F. Hoffmann-La Roche and Others
Judgment of 23/01/2018